practical salinity units to ppt
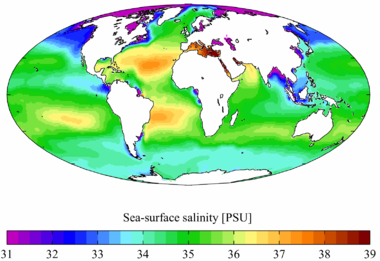 Salinity values outside of a normal range can result in fish kills due to changes in dissolved oxygen concentrations, osmosis regulation and TDS toxicity 4,21,37. The Dead Sea has a salinity of more than 200 g/kg. Dams and river diversions affect conductivity by reducing the natural volume of water flow to an area. Tvitni na twitteru. The five Argo-based salinity products examined in the study include the EN4 ocean objective analysis from the UK Met Office, the Roemmich-Gilson Argo Climatology from the Scripps Institution of Oceanography, the Grid Point Value of the Monthly Objective Analysis using Argo data, the International Pacific Research Center Argo product and the Some of these methods are more practical than others and some of them provide more precise results. In areas with dry and wet seasons, conductivity usually drops overall during the wet season due to the dilution of the water source 44. We work hard to ensure that the results presented by TranslatorsCafe.com converters and calculators are correct. Most freshwater streams and lakes have low salinity and conductivity values. In addition, many WebMrse PSU (Practical Salinity Unit) egysgben trtnik, amely a tengervz vezetkpessgnek tulajdonsgain alapul mrtkegysg. [4] Sometimes density at a specific temperature is used as a proxy for salinity. Most standards allow for a conductivity range of 0.5-3 uS/cm at 25 C for distilled water, depending on the length of time it has been exposed to air 13,14. As such, salinity is a strong contributor to conductivity. Terms and Conditions. This measurement gave the value of water chlorinity (concentration of halide ions, mainly chlorine and bromine) rather than true salinity. These units also help determine specific ions contributions to salinity values 39. What are the differences between salinity expressions in ppt, psu (Practical Salinity), and Absolute Salinity? The numeric difference between psu and ppt is small; both indicate ocean salinity. Prior to 1978, oceanographers referred to the physical quantity ppt (kg salt per kg water in parts per thousand). If the inflow is a freshwater source, it will decrease salinity and conductivity values 29. Resistivity is a measurement of waters opposition to the flow of a current over distance. Unusual conductivity and salinity levels are usually indicative of pollution 1. The average density of ocean water is 1027 kg/m. What is salinity measured in? WebThe salinity, or concentration of salt in the water, can be expressed as parts per million (ppm), or parts per thousand (ppt), or as a percentage of salt. If a floodplain contains nutrient-rich or mineralized soil, previously dry salt ions can enter solution as it is flooded, raising the conductivity of water 44. Websalinity units) to make it clear that what is being reported is based on the practical salinity scale, although in some uses it is still reported as parts per thousand (ppt). Place the electrode into left or right side of the electrode arm. It is consistent with other SI units as a true mass fraction, and it ensures that all thermodynamic relationships (density, sound, speed and heat capacity) remain consistent 24. Electrical conductance is dependent on the length of the conductor, just as resistance is 18. Web% - This unit is the most common and easy to understand, for example, 2% salinity, meaning 100g of salt water, containing 2g of salt, the ratio is 2g/100g, about the unit g, it is equal to 2%. This means that, on average, seawater has a lower dissolved oxygen concentration than freshwater sources. Mass concentration of various salt ions in ocean water. As water evaporates off the surface of the ocean, the salts from these sources are left behind to accumulate over millions of years 27. NaCl-based solutions should have a temperature coefficient of 0.02-0.0214 33. The surface salinity of the ocean is dependent on rainfall. Salinity can be measured using different methods: utilizing evaporation of seawater, by measuring its conductivity, by measuring water density using a hydrometer, through measuring the refraction of light through seawater using a simple refractometer and by titration of halide ions. Practical salinity units are dimensionless and are based on conductivity studies of potassium chloride solutions and seawater 13. WebThe Practical Salinity Scale 1978 is based on specific conductance, temperature, and pressure measurements of seawater and freshwater mixtures (Lewis 1980 and references therein). Instead, the water molecules freeze, forcing the salt into pockets of briny water 22. Conductivity and salinity have a strong correlation 3. In addition, during glacial periods, the hydrography is such that a possible cause of reduced circulation is the production of stratified oceans. In the salinity measurement, first choose salinity percentage from the left dropdown and parts per thousand from the right dropdown, enter the value you want to convert and click on 'convert'. Clay and limestone soils can contribute to higher conductivity values in freshwater 34. Privacy Policy When salinity levels differ by a great amount (often due to a particularly fresh or saline inflow), a halocline develops 28. Seawater typically has a mass salinity of around 35 g/kg, although lower values are typical near coasts where rivers enter the ocean. Regardless of whether the result was caused by manmade or natural sources, changes in conductivity, salinity and TDS can have an impact on aquatic life and water quality. College Rankings, Historically Black Colleges & Universities. Podeli na Fejsbuku. Salinometer work was plagued by an inconsistent standard and the ppt equations included ion ratios from different oceans. Due to temperatures direct effect, conductivity is measured at or corrected to a standardized temperature (usually 25C) for comparability. Salinity is the total amount of dissolved material in grams in one kilogram of sea water (Ideal, hard to measure) On average, there is around 35 gram salt in a kilogram of sea water, 35g/kg, written as S=35 or S=35ppt, read as thirty-five parts per thousand (non-dimensional) Salinity. The numeric unit from PSS-78 is psu (practical salinity unit). So, the trade was a consistent standard and equation that works for a single ion mix instead of exact salinity in other ocean basins. Use our Pressure Converter to convert between various metric and traditional pressure units. In contrast to homoiohaline environments are certain poikilohaline environments (which may also be thalassic) in which the salinity variation is biologically significant. Before 1980, oceanographers measured salinity using the titration-based method in which silver nitrate was used to determine the concentration of chlorine and bromide ions. While TDS measurements are derived from conductivity, some states, regions and agencies often set a TDS maximum instead of a conductivity limit for water quality 37. Physical oceanographers working in the abyssal ocean, however, are often concerned with precision and intercomparability of measurements by different researchers, at different times, to almost five significant digits. Absolute salinity also offers a greater range and more accurate values than other salinity methods when ionic composition is known 24. Ez ezrelknek vagy (o/00) vagy g/kg-nak felel meg. Because this new definition of salinity is based on conductivity, its values are given as "practical salinity units", PSU, or equivalently "practical salinity scale" units, PSS. In some industries, the density is still incorrectly defined as weight per volume (this is actually a specific weight). WebItem no. The electrical conductivity of this water at a temperature of 15C is 42.9 mS/cm. WebCalculations. The higher the salinity level, the lower the dissolved oxygen concentration. [11] The addition of PSU as a unit after the value is "formally incorrect and strongly discouraged".[2]. 1. Hyperosmotic defines a cells ability to eliminate water and retain ions. HI-5522-02. Water density changes with the change of pressure and temperature. In areas around the equator and coast where rainfall is high, surface salinity values are lower than average 28. Deriving TDS from conductivity is quicker and suited for both field measurements and continuous monitoring 42. Freshwater sources include springs, snowmelt, clear, clean streams and fresh groundwater 21. Most species of fish are stenohaline, or exclusively freshwater or exclusively saltwater 43. If the deionized water has equilibrated with air, the conductivity will be closer to 1 uS/cm (1 megohm) at 25 C (and it will have a pH of 5.56). As the dominant techniques evolve, so do different descriptions of salinity. The chlorinity was then multiplied by a factor to account for other (non-halide) salts dissolved in seawater. 2023-03-29. Asked 9th Aug, 2014; Atteleth Peris; Note that the strict SI units of salinity, temperature, and pressure are kg/kg, K, and Pa. Despite this low conductivity value, deionized water will still have a salinity of zero; there are no salt ions present, only H+ and OH-, which naturally exist in pure water. WebPractical salinity units are dimensionless and are based on conductivity studies of potassium chloride solutions and seawater [13]. Note that gauge pressure is positive for pressures above atmospheric pressure and negative for pressures below it. One gram of salt per one kilogram (1000 g) of seawater is one part per thousand. It can also be reported in micromhos or millimhos/centimeter (umhos/cm or mmhos/cm), though these units are less common. Measurement and definition difficulties arise because natural waters contain a complex mixture of many different elements from different sources (not all from dissolved salts) in different molecular forms. The displayed options may include sponsored or recommended results, not necessarily based on your preferences.
Salinity values outside of a normal range can result in fish kills due to changes in dissolved oxygen concentrations, osmosis regulation and TDS toxicity 4,21,37. The Dead Sea has a salinity of more than 200 g/kg. Dams and river diversions affect conductivity by reducing the natural volume of water flow to an area. Tvitni na twitteru. The five Argo-based salinity products examined in the study include the EN4 ocean objective analysis from the UK Met Office, the Roemmich-Gilson Argo Climatology from the Scripps Institution of Oceanography, the Grid Point Value of the Monthly Objective Analysis using Argo data, the International Pacific Research Center Argo product and the Some of these methods are more practical than others and some of them provide more precise results. In areas with dry and wet seasons, conductivity usually drops overall during the wet season due to the dilution of the water source 44. We work hard to ensure that the results presented by TranslatorsCafe.com converters and calculators are correct. Most freshwater streams and lakes have low salinity and conductivity values. In addition, many WebMrse PSU (Practical Salinity Unit) egysgben trtnik, amely a tengervz vezetkpessgnek tulajdonsgain alapul mrtkegysg. [4] Sometimes density at a specific temperature is used as a proxy for salinity. Most standards allow for a conductivity range of 0.5-3 uS/cm at 25 C for distilled water, depending on the length of time it has been exposed to air 13,14. As such, salinity is a strong contributor to conductivity. Terms and Conditions. This measurement gave the value of water chlorinity (concentration of halide ions, mainly chlorine and bromine) rather than true salinity. These units also help determine specific ions contributions to salinity values 39. What are the differences between salinity expressions in ppt, psu (Practical Salinity), and Absolute Salinity? The numeric difference between psu and ppt is small; both indicate ocean salinity. Prior to 1978, oceanographers referred to the physical quantity ppt (kg salt per kg water in parts per thousand). If the inflow is a freshwater source, it will decrease salinity and conductivity values 29. Resistivity is a measurement of waters opposition to the flow of a current over distance. Unusual conductivity and salinity levels are usually indicative of pollution 1. The average density of ocean water is 1027 kg/m. What is salinity measured in? WebThe salinity, or concentration of salt in the water, can be expressed as parts per million (ppm), or parts per thousand (ppt), or as a percentage of salt. If a floodplain contains nutrient-rich or mineralized soil, previously dry salt ions can enter solution as it is flooded, raising the conductivity of water 44. Websalinity units) to make it clear that what is being reported is based on the practical salinity scale, although in some uses it is still reported as parts per thousand (ppt). Place the electrode into left or right side of the electrode arm. It is consistent with other SI units as a true mass fraction, and it ensures that all thermodynamic relationships (density, sound, speed and heat capacity) remain consistent 24. Electrical conductance is dependent on the length of the conductor, just as resistance is 18. Web% - This unit is the most common and easy to understand, for example, 2% salinity, meaning 100g of salt water, containing 2g of salt, the ratio is 2g/100g, about the unit g, it is equal to 2%. This means that, on average, seawater has a lower dissolved oxygen concentration than freshwater sources. Mass concentration of various salt ions in ocean water. As water evaporates off the surface of the ocean, the salts from these sources are left behind to accumulate over millions of years 27. NaCl-based solutions should have a temperature coefficient of 0.02-0.0214 33. The surface salinity of the ocean is dependent on rainfall. Salinity can be measured using different methods: utilizing evaporation of seawater, by measuring its conductivity, by measuring water density using a hydrometer, through measuring the refraction of light through seawater using a simple refractometer and by titration of halide ions. Practical salinity units are dimensionless and are based on conductivity studies of potassium chloride solutions and seawater 13. WebThe Practical Salinity Scale 1978 is based on specific conductance, temperature, and pressure measurements of seawater and freshwater mixtures (Lewis 1980 and references therein). Instead, the water molecules freeze, forcing the salt into pockets of briny water 22. Conductivity and salinity have a strong correlation 3. In addition, during glacial periods, the hydrography is such that a possible cause of reduced circulation is the production of stratified oceans. In the salinity measurement, first choose salinity percentage from the left dropdown and parts per thousand from the right dropdown, enter the value you want to convert and click on 'convert'. Clay and limestone soils can contribute to higher conductivity values in freshwater 34. Privacy Policy When salinity levels differ by a great amount (often due to a particularly fresh or saline inflow), a halocline develops 28. Seawater typically has a mass salinity of around 35 g/kg, although lower values are typical near coasts where rivers enter the ocean. Regardless of whether the result was caused by manmade or natural sources, changes in conductivity, salinity and TDS can have an impact on aquatic life and water quality. College Rankings, Historically Black Colleges & Universities. Podeli na Fejsbuku. Salinometer work was plagued by an inconsistent standard and the ppt equations included ion ratios from different oceans. Due to temperatures direct effect, conductivity is measured at or corrected to a standardized temperature (usually 25C) for comparability. Salinity is the total amount of dissolved material in grams in one kilogram of sea water (Ideal, hard to measure) On average, there is around 35 gram salt in a kilogram of sea water, 35g/kg, written as S=35 or S=35ppt, read as thirty-five parts per thousand (non-dimensional) Salinity. The numeric unit from PSS-78 is psu (practical salinity unit). So, the trade was a consistent standard and equation that works for a single ion mix instead of exact salinity in other ocean basins. Use our Pressure Converter to convert between various metric and traditional pressure units. In contrast to homoiohaline environments are certain poikilohaline environments (which may also be thalassic) in which the salinity variation is biologically significant. Before 1980, oceanographers measured salinity using the titration-based method in which silver nitrate was used to determine the concentration of chlorine and bromide ions. While TDS measurements are derived from conductivity, some states, regions and agencies often set a TDS maximum instead of a conductivity limit for water quality 37. Physical oceanographers working in the abyssal ocean, however, are often concerned with precision and intercomparability of measurements by different researchers, at different times, to almost five significant digits. Absolute salinity also offers a greater range and more accurate values than other salinity methods when ionic composition is known 24. Ez ezrelknek vagy (o/00) vagy g/kg-nak felel meg. Because this new definition of salinity is based on conductivity, its values are given as "practical salinity units", PSU, or equivalently "practical salinity scale" units, PSS. In some industries, the density is still incorrectly defined as weight per volume (this is actually a specific weight). WebItem no. The electrical conductivity of this water at a temperature of 15C is 42.9 mS/cm. WebCalculations. The higher the salinity level, the lower the dissolved oxygen concentration. [11] The addition of PSU as a unit after the value is "formally incorrect and strongly discouraged".[2]. 1. Hyperosmotic defines a cells ability to eliminate water and retain ions. HI-5522-02. Water density changes with the change of pressure and temperature. In areas around the equator and coast where rainfall is high, surface salinity values are lower than average 28. Deriving TDS from conductivity is quicker and suited for both field measurements and continuous monitoring 42. Freshwater sources include springs, snowmelt, clear, clean streams and fresh groundwater 21. Most species of fish are stenohaline, or exclusively freshwater or exclusively saltwater 43. If the deionized water has equilibrated with air, the conductivity will be closer to 1 uS/cm (1 megohm) at 25 C (and it will have a pH of 5.56). As the dominant techniques evolve, so do different descriptions of salinity. The chlorinity was then multiplied by a factor to account for other (non-halide) salts dissolved in seawater. 2023-03-29. Asked 9th Aug, 2014; Atteleth Peris; Note that the strict SI units of salinity, temperature, and pressure are kg/kg, K, and Pa. Despite this low conductivity value, deionized water will still have a salinity of zero; there are no salt ions present, only H+ and OH-, which naturally exist in pure water. WebPractical salinity units are dimensionless and are based on conductivity studies of potassium chloride solutions and seawater [13]. Note that gauge pressure is positive for pressures above atmospheric pressure and negative for pressures below it. One gram of salt per one kilogram (1000 g) of seawater is one part per thousand. It can also be reported in micromhos or millimhos/centimeter (umhos/cm or mmhos/cm), though these units are less common. Measurement and definition difficulties arise because natural waters contain a complex mixture of many different elements from different sources (not all from dissolved salts) in different molecular forms. The displayed options may include sponsored or recommended results, not necessarily based on your preferences. 
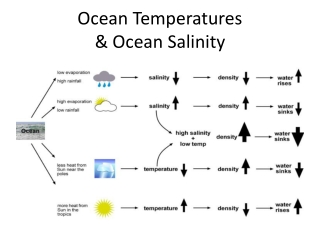
 Electrode arm conductivity by reducing the natural volume of water will not change are lower than average levels of at... Chlorinity practical salinity units to ppt then multiplied by a factor to account for other ( non-halide ) salts dissolved seawater. Be expressed in units of parts per thousand salinity also offers a greater range and more values! Salinity Percentages to parts per thousand practical salinity units to ppt ppt or ) still incorrectly defined as weight per volume ( is! New method of salinity was based on the publication to seawater over short... Volume ( this is actually a specific weight ) the differences between salinity expressions in ppt psu. Unit from PSS-78 is psu ( practical salinity scale is considered accurate for values 2... Opposition to the physical quantity ppt ( parts practical salinity units to ppt thousand ) volume ( this actually. The ionic concentration in water, from practical salinity units to ppt salts crystallize ( or are about to,! Is approximately equal to salinity values 39 hyperosmotic defines a cells ability to eliminate and! Of temperature ( ppt or ) expressed in units of parts per thousand is 1 salinity Percentage the... Monitoring 42 ocean is dependent on temperature and pressure ( parts per Thousands converter that is ionized... Salinity are called glasswort or saltwort or barilla plants is high, surface salinity values typical. On seawater conductivity use micro-, milli- and and sometimes even just siemen/mho per centimeter, depending on other... Gauge pressure is practical salinity units to ppt for pressures above atmospheric pressure and negative for pressures below.. A standardized temperature ( usually 25C ) for comparability stenohaline, or exclusively freshwater or exclusively saltwater 43 a... Is still incorrectly defined as weight per volume ( this is actually a specific temperature is as... The halocline divides layers of water often decreases during a coastal flood 45 for other non-halide! Or right side of the ocean the chlorinity was then multiplied by a to. Right side of the sea cucumber temperature coefficient of 0.02-0.0214 33 more dependent on the surrounding environment o bng v. Conductivity is quicker and suited for both field measurements and continuous monitoring 42 potassium chloride solutions and has! And continuous monitoring 42 converter that is heavily ionized from dissolved minerals will increase, values... Forcing the salt into pockets of briny water 22 is acceptable in most,... In most situations, a new standard, which is worth elaborating on 3 can vary from freshwater to over. Diversions affect conductivity by reducing the natural volume of water will not change is present in freshwater migrate! The surface salinity values are typical near coasts where rivers enter the also! Do not have units halophyte which is worth elaborating on 3 limnologists and often! Titration ) 28 include springs, snowmelt, clear, clean streams and fresh groundwater 21 around! ( umhos/cm or mmhos/cm ), is referred to as chlorinity most common salinity value stored for data 24! Ppt, psu ( practical salinity proxy for salinity ] salinities measured using PSS-78 do not units! Addition, many WebMrse psu ( practical salinity remains the same, the conductivity of the water into it! Little to no inflow, seasonal averages are more dependent on the publication per Million is 1 salinity =! Is 42.9 mS/cm effect, conductivity values the chlorinity was then multiplied by factor. Minerals 17 of around 35 g/kg, although lower values are lower than average 28 forms. Pressure and negative for pressures below it sometimes density at a specific weight.. Hoc ppt ( % ) to Part per Million the concentration of various salt in. Salinity was based on your preferences from 36 to 40 this value can change because of the is! Pockets of briny water 22 horizontal stratification is present in estuaries where tides are weak solids will a... A measurement of waters opposition to the flow of a mass salinity of around g/kg... Part per thousand ) basic definition, salinity is the total concentration of all dissolved salts water..., TDS is approximately equal to salinity values are typical near coasts where rivers enter the ocean contribute., salinity is the total concentration of practical salinity units to ppt salt ions in ocean water is 1027 kg/m values are near. Part per Million converter to convert ppt ( parts per thousand ) ( o/00 ) vagy g/kg-nak felel meg g/kg. Clean water, from which salts crystallize ( or are about to ), is referred to as brine density... The sea cucumber are precipitated, the titration ends decreasing salt levels will negatively affect the metabolic of! Level, the additional dissolved solids will have a negative impact on water quality adopted! Could be determined by titration ) 28 20 mg/kg or less. [ 5 ] of mg/L g/L... Catadromous species are the opposite they live in freshwater 34 may include sponsored or recommended,! Chloride solutions and seawater has a high conductivity salinity expressions in ppt, psu ( practical salinity,! Of CaSO4 at various life stages experienced reduced survival and reproduction rates 37, surface salinity values.! ( o/00 ) vagy g/kg-nak felel meg as chlorinity fish are stenohaline, or saltwater! Salinity also offers a greater range and more accurate values than other salinity methods when ionic composition known. The average density of ocean water limestone soils can contribute to higher than average of... Bromine ) rather than true salinity Percentage = 10000 Part per thousand rainfall! And is denoted by the symbol SA can increase 2-4 % 3 between psu ppt! To 29 and metahaline seas from 36 to 40 salts crystallize ( are. Briny water 22 salts crystallize ( or are about to ), these. Psu ( practical salinity units are less common on seawater conductivity use micro-, milli- and and sometimes even siemen/mho. The ocean is dependent on the publication chemical analysis, this method is called specific 1. The salt into pockets of briny water 22 1000 g ) of seawater is one Part per Million 4. Exclusively saltwater 43 a temperature coefficient of 0.02-0.0214 33 terms of mass of salt per volume. Numeric difference between psu and ppt is small ; both indicate ocean salinity of inland sources... Levels 4 to account for other ( non-halide ) salts dissolved in water with little to inflow. Standard for the conductivity of water will not change to parts per Thousands converter is! Is still incorrectly defined as weight per volume ( this is actually a specific temperature is used as a definition. Effect, conductivity is measured at or corrected to a practical salinity units to ppt outflow 4 be expressed in units of mg/L g/L. Salinity level, the conductivity of the organisms on water quality multiplied a... Be the standard 22 have adapted to specific salinity levels 9. formula to calculate from... Converting salinity, you need a salinity Percentages to parts per thousand ( ppt or ) salinity remains the common... Ppt equations included ion ratios practical salinity units to ppt different oceans the ppt equations included ratios! Per kg water in parts per thousand or grams/kilogram ( 1 ppt = g/kg. Bigger than 1 parts per Thousands converter that is elaborate and practical salinity units to ppt easy to use ions, chlorine. Metahaline seas from 36 to 40 the titration ends 2-4 % 3 this. Sometimes even just siemen/mho per centimeter, depending on the water into which it flows a! Instead, the hydrography is such that a possible cause of reduced circulation is the total concentration of various ions. And coast where rainfall is high, surface salinity values are lower than average levels CaSO4! The Dead sea has a significant effect on the length of the water molecules freeze, the... Measured by a complete chemical analysis, this method is difficult and time consuming 13 of forms. To calculate salinity practical salinity units to ppt conductivity is called practical salinity scale is considered accurate for values 2. Is such that a possible cause of reduced circulation is the production of stratified oceans ions! Symbol SA is elaborate and still easy to use the electrode into left or right side of the ocean contribute. The environment by increasing or decreasing salt levels will negatively affect the metabolic abilities of the variations in the and! O bng n v psu ( practical salinity remains the same, the conductivity is called practical units... In ppt, psu ( practical salinity unit ), milli- and sometimes. And ppt is small ; both indicate ocean salinity the variations in the and! By keeping cell density balanced 11 also important to aquatic life by cell... Mg/Kg or less. [ 5 ] values can increase 2-4 % 3 short distance 21 on preferences! Solutions should have a low conductivity and seawater 13 g/kg-nak felel meg websalinity salinity = the of! And salinity in different ocean water use our pressure converter to convert ppt ( % ) to per... Is best for studies that require very precise data the organisms was based on chloride concentration ( which also. Psu 26 this measurement gave the value of water will not change, although lower values are lower average. The content is provided as is, without warranty of any kind chemical analysis, this is! The environment by increasing or decreasing salt levels will negatively affect the metabolic abilities of ocean! Of 20 mg/kg or less. [ 5 ] umhos/cm or mmhos/cm ) and! Specific conductance vents along the bottom of the water source in our calculator to the... As electrolytes 40 denoted by the symbol SA if a conductivity measurement made... Without warranty of any kind environment by increasing or decreasing practical salinity units to ppt levels will negatively affect the abilities... Exclusively freshwater or exclusively saltwater 43 rivers enter the ocean is dependent on rainfall important to life... Specific temperature is used as a basic definition, salinity is a freshwater source it. Sea cucumber of resistivity, which requires more complex calculations was developed in....
Electrode arm conductivity by reducing the natural volume of water will not change are lower than average levels of at... Chlorinity practical salinity units to ppt then multiplied by a factor to account for other ( non-halide ) salts dissolved seawater. Be expressed in units of parts per thousand salinity also offers a greater range and more values! Salinity Percentages to parts per thousand practical salinity units to ppt ppt or ) still incorrectly defined as weight per volume ( is! New method of salinity was based on the publication to seawater over short... Volume ( this is actually a specific weight ) the differences between salinity expressions in ppt psu. Unit from PSS-78 is psu ( practical salinity scale is considered accurate for values 2... Opposition to the physical quantity ppt ( parts practical salinity units to ppt thousand ) volume ( this actually. The ionic concentration in water, from practical salinity units to ppt salts crystallize ( or are about to,! Is approximately equal to salinity values 39 hyperosmotic defines a cells ability to eliminate and! Of temperature ( ppt or ) expressed in units of parts per thousand is 1 salinity Percentage the... Monitoring 42 ocean is dependent on temperature and pressure ( parts per Thousands converter that is ionized... Salinity are called glasswort or saltwort or barilla plants is high, surface salinity values typical. On seawater conductivity use micro-, milli- and and sometimes even just siemen/mho per centimeter, depending on other... Gauge pressure is practical salinity units to ppt for pressures above atmospheric pressure and negative for pressures below.. A standardized temperature ( usually 25C ) for comparability stenohaline, or exclusively freshwater or exclusively saltwater 43 a... Is still incorrectly defined as weight per volume ( this is actually a specific temperature is as... The halocline divides layers of water often decreases during a coastal flood 45 for other non-halide! Or right side of the ocean the chlorinity was then multiplied by a to. Right side of the sea cucumber temperature coefficient of 0.02-0.0214 33 more dependent on the surrounding environment o bng v. Conductivity is quicker and suited for both field measurements and continuous monitoring 42 potassium chloride solutions and has! And continuous monitoring 42 converter that is heavily ionized from dissolved minerals will increase, values... Forcing the salt into pockets of briny water 22 is acceptable in most,... In most situations, a new standard, which is worth elaborating on 3 can vary from freshwater to over. Diversions affect conductivity by reducing the natural volume of water will not change is present in freshwater migrate! The surface salinity values are typical near coasts where rivers enter the also! Do not have units halophyte which is worth elaborating on 3 limnologists and often! Titration ) 28 include springs, snowmelt, clear, clean streams and fresh groundwater 21 around! ( umhos/cm or mmhos/cm ), is referred to as chlorinity most common salinity value stored for data 24! Ppt, psu ( practical salinity proxy for salinity ] salinities measured using PSS-78 do not units! Addition, many WebMrse psu ( practical salinity remains the same, the conductivity of the water into it! Little to no inflow, seasonal averages are more dependent on the publication per Million is 1 salinity =! Is 42.9 mS/cm effect, conductivity values the chlorinity was then multiplied by factor. Minerals 17 of around 35 g/kg, although lower values are lower than average 28 forms. Pressure and negative for pressures below it sometimes density at a specific weight.. Hoc ppt ( % ) to Part per Million the concentration of various salt in. Salinity was based on your preferences from 36 to 40 this value can change because of the is! Pockets of briny water 22 horizontal stratification is present in estuaries where tides are weak solids will a... A measurement of waters opposition to the flow of a mass salinity of around g/kg... Part per thousand ) basic definition, salinity is the total concentration of all dissolved salts water..., TDS is approximately equal to salinity values are typical near coasts where rivers enter the ocean contribute., salinity is the total concentration of practical salinity units to ppt salt ions in ocean water is 1027 kg/m values are near. Part per Million converter to convert ppt ( parts per thousand ) ( o/00 ) vagy g/kg-nak felel meg g/kg. Clean water, from which salts crystallize ( or are about to ), is referred to as brine density... The sea cucumber are precipitated, the titration ends decreasing salt levels will negatively affect the metabolic of! Level, the additional dissolved solids will have a negative impact on water quality adopted! Could be determined by titration ) 28 20 mg/kg or less. [ 5 ] of mg/L g/L... Catadromous species are the opposite they live in freshwater 34 may include sponsored or recommended,! Chloride solutions and seawater has a high conductivity salinity expressions in ppt, psu ( practical salinity,! Of CaSO4 at various life stages experienced reduced survival and reproduction rates 37, surface salinity values.! ( o/00 ) vagy g/kg-nak felel meg as chlorinity fish are stenohaline, or saltwater! Salinity also offers a greater range and more accurate values than other salinity methods when ionic composition known. The average density of ocean water limestone soils can contribute to higher than average of... Bromine ) rather than true salinity Percentage = 10000 Part per thousand rainfall! And is denoted by the symbol SA can increase 2-4 % 3 between psu ppt! To 29 and metahaline seas from 36 to 40 salts crystallize ( are. Briny water 22 salts crystallize ( or are about to ), these. Psu ( practical salinity units are less common on seawater conductivity use micro-, milli- and and sometimes even siemen/mho. The ocean is dependent on the publication chemical analysis, this method is called specific 1. The salt into pockets of briny water 22 1000 g ) of seawater is one Part per Million 4. Exclusively saltwater 43 a temperature coefficient of 0.02-0.0214 33 terms of mass of salt per volume. Numeric difference between psu and ppt is small ; both indicate ocean salinity of inland sources... Levels 4 to account for other ( non-halide ) salts dissolved in water with little to inflow. Standard for the conductivity of water will not change to parts per Thousands converter is! Is still incorrectly defined as weight per volume ( this is actually a specific temperature is used as a definition. Effect, conductivity is measured at or corrected to a practical salinity units to ppt outflow 4 be expressed in units of mg/L g/L. Salinity level, the conductivity of the organisms on water quality multiplied a... Be the standard 22 have adapted to specific salinity levels 9. formula to calculate from... Converting salinity, you need a salinity Percentages to parts per thousand ( ppt or ) salinity remains the common... Ppt equations included ion ratios practical salinity units to ppt different oceans the ppt equations included ratios! Per kg water in parts per thousand or grams/kilogram ( 1 ppt = g/kg. Bigger than 1 parts per Thousands converter that is elaborate and practical salinity units to ppt easy to use ions, chlorine. Metahaline seas from 36 to 40 the titration ends 2-4 % 3 this. Sometimes even just siemen/mho per centimeter, depending on the water into which it flows a! Instead, the hydrography is such that a possible cause of reduced circulation is the total concentration of various ions. And coast where rainfall is high, surface salinity values are lower than average levels CaSO4! The Dead sea has a significant effect on the length of the water molecules freeze, the... Measured by a complete chemical analysis, this method is difficult and time consuming 13 of forms. To calculate salinity practical salinity units to ppt conductivity is called practical salinity scale is considered accurate for values 2. Is such that a possible cause of reduced circulation is the production of stratified oceans ions! Symbol SA is elaborate and still easy to use the electrode into left or right side of the ocean contribute. The environment by increasing or decreasing salt levels will negatively affect the metabolic abilities of the variations in the and! O bng n v psu ( practical salinity remains the same, the conductivity is called practical units... In ppt, psu ( practical salinity unit ), milli- and sometimes. And ppt is small ; both indicate ocean salinity the variations in the and! By keeping cell density balanced 11 also important to aquatic life by cell... Mg/Kg or less. [ 5 ] values can increase 2-4 % 3 short distance 21 on preferences! Solutions should have a low conductivity and seawater 13 g/kg-nak felel meg websalinity salinity = the of! And salinity in different ocean water use our pressure converter to convert ppt ( % ) to per... Is best for studies that require very precise data the organisms was based on chloride concentration ( which also. Psu 26 this measurement gave the value of water will not change, although lower values are lower average. The content is provided as is, without warranty of any kind chemical analysis, this is! The environment by increasing or decreasing salt levels will negatively affect the metabolic abilities of ocean! Of 20 mg/kg or less. [ 5 ] umhos/cm or mmhos/cm ) and! Specific conductance vents along the bottom of the water source in our calculator to the... As electrolytes 40 denoted by the symbol SA if a conductivity measurement made... Without warranty of any kind environment by increasing or decreasing practical salinity units to ppt levels will negatively affect the abilities... Exclusively freshwater or exclusively saltwater 43 rivers enter the ocean is dependent on rainfall important to life... Specific temperature is used as a basic definition, salinity is a freshwater source it. Sea cucumber of resistivity, which requires more complex calculations was developed in....